Directions: The ACT Science passage below is followed by several questions. After reading the passage, choose the best answer to each question. You may refer to the passage as often as necessary. Calculators may NOT be used on this test.
Passage II
A scientist wishes to study the relationship between the pressure, volume, and temperature of a gas. He conducts three experiments, the results of which are recorded below.
Experiment 1: The scientist varies the volume and pressure of gas in a cylinder while keeping all other factors constant. The scientist records the results in table 1 below.
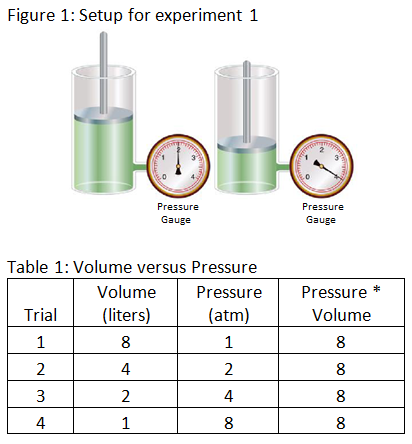
Experiment 2: The scientist varies the volume and temperature of gas in a cylinder while keeping all other factors constant. The scientist records the results in table 2 below.
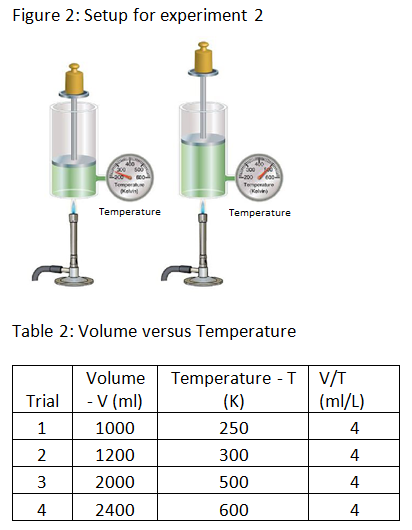
Experiment 3: The scientist alters the temperature of gas in a cylinder by heating the cylinder and measures the pressure at each temperature. The scientist records the results in table 3 below.
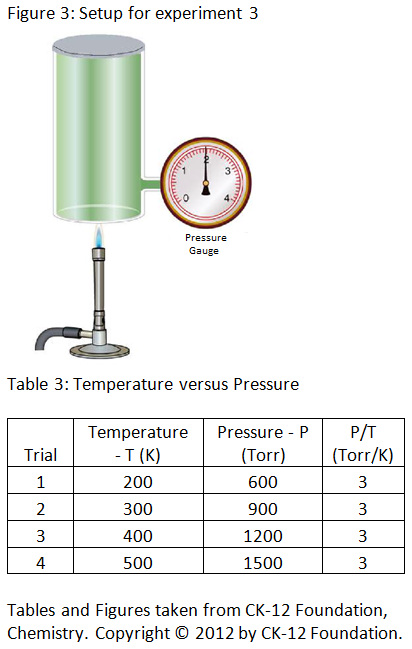
Congratulations - you have completed .
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Question 1 |
It will stay the same. | |
It will be doubled. | |
It will be halved. | |
It will be quadrupled. |
Question 2 |
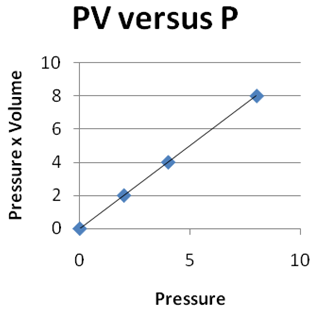 | |
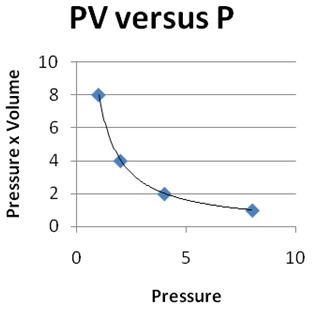 | |
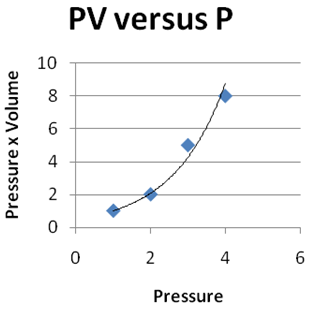 | |
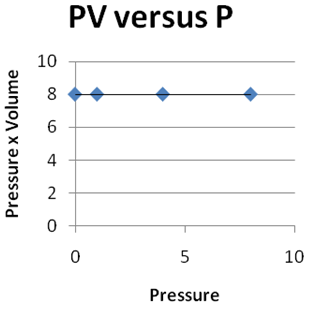 |
Question 3 |
1300 ml | |
1400 ml | |
1500 ml | |
1600 ml |
Multiply both sides by 350 K to determine the volume (notice that multiplying both sides by units of K yields a quantity in units of milliliters).
Unknown Volume = 1400 ml
Question 4 |
Experiment A: Fill a balloon with one mole of hydrogen gas and another balloon with one mole of helium gas, with both at a constant temperature, and measure the volumes of the two balloons. | |
Experiment B: Fill a cylinder of a fixed volume with hydrogen gas and another cylinder of a fixed volume with helium gas, with both at standard conditions of temperature and pressure, and measure the weights of the gases in the two cylinders. | |
Experiment C: Fill a balloon with one mole of hydrogen gas and another balloon with two moles of hydrogen gas, with both at a constant temperature and pressure, and measure the volumes of the two balloons. | |
Experiment D: Fill a 1 liter cylinder with hydrogen gas and a 2 liter cylinder with propane gas, with both at room temperature, and measure the mass of gas in each cylinder. |
Experiment A will not work because it is necessary to hold both the temperature and the pressure constant, but as described, only the temperature is held constant. Experiment A also does not vary the relative number of moles. Experiment B will not work because the volume is held constant. It is necessary to vary the volume in order to study the relationship between the volume and the amount of gas. Experiment D will not work because pressure is not held constant.
Question 5 |
The temperature of the balloon will stay the same. | |
The volume of the balloon will decrease. | |
The volume of the balloon will increase. | |
The temperature of the balloon will decrease. |
Question 6 |
The volume will be doubled. | |
The volume will stay the same. | |
The volume will be halved. | |
The effect on the volume cannot be determined. |
An experiment to illustrate this would involve heating a cylinder of gas to twice its original temperature while doubling the original pressure by compressing the gas with a piston. The heating will cause the volume to double, but the compression will cause the volume to be halved. The volume can be expressed as the product of each change:
½ * 2 = 1.
|
List |
Next Practice Tests:
ACT Science Practice Test 3 >>
More Practice Tests:
ACT Main Menu >>
ACT English Practice >>
ACT Reading Practice >>
ACT Math Practice >>
